
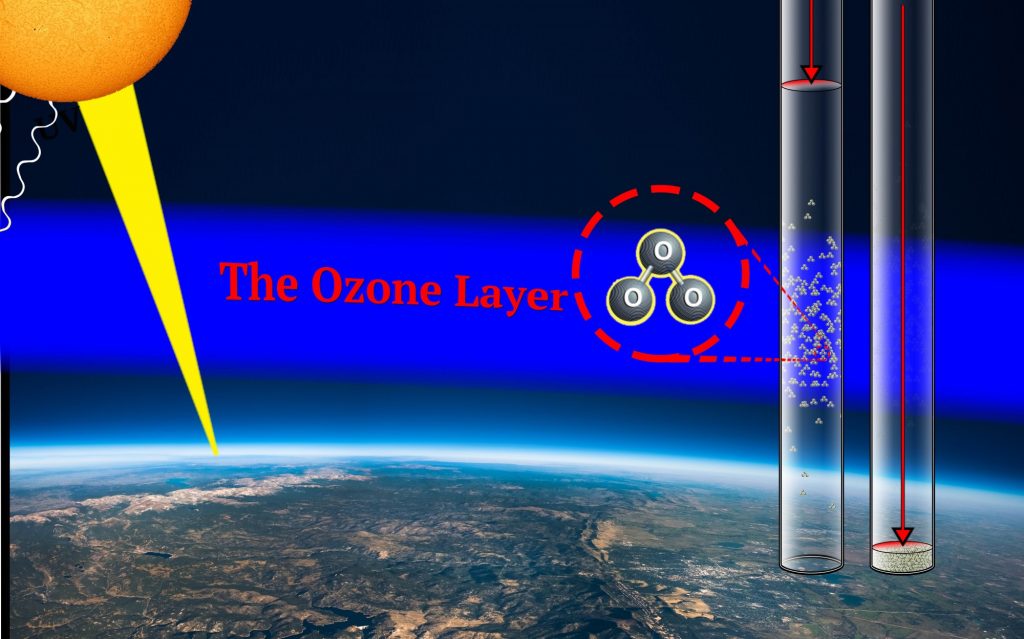
The Ozone Layer is the name given to large concentrations of naturally occurring ozone located high in the stratosphere between 35,000 – 160,000 feet. The ozone layer is important to life on Earth because of its ability to filter out harmful doses of ultraviolet radiation from the Sun that damage DNA in plants and animals and is the primary cause of skin cancer in humans. In this way, the ozone layer protects the Earth as a natural, global sunscreen.
In 1974 scientists Sherwood Rowland and Mario Molina published an article in Nature warning the world that use of popular chemicals called chlorofluorocarbons (CFC’s) was transporting huge quantities of destructive chlorine high in to the atmosphere. Their discovery was met with intense pushback from representatives of the aerosol and halocarbon industries who were critical of their calls to action. Though their research did eventually lead to some restrictions on CFC release in the late 1970s and early 1980s, it was not until 1985 when Joseph Farman and colleagues noted a drastic depletion of ozone above Antarctica that we learned even Rowland and Molina had underestimated the dangers.
In 1995 Sherwood Rowland and Mario Molina were awarded the Nobel Prize in Chemistry for their discovery.

In response to this discovery, leaders of the World came together to ratify the Montreal Protocol on Substances that Deplete the Ozone Layer. This international treaty, signed in to action in 1987, phases out production of these extremely useful, but ultimately harmful Ozone-Depleting Substances. Ratified by all 197 members, the Montreal Protocol was the first universally ratified treaty in United Nations history and an incredible example of scientific discovery directing international policy.
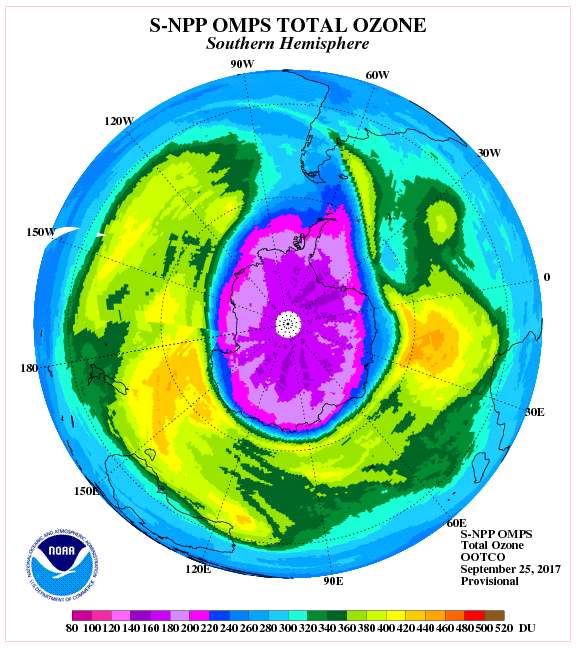
This large, thin spot in the ozone layer came to be known as the “Ozone Hole.” Since 1986, NOAA’s Ozone & Water Vapor Group have performed regular launches of high altitude balloons from the South Pole with instruments to measure this destruction, and eventual recovery, of the ozone layer above Antarctica.


The small insulated enclosure attached to the bottom of the balloon carries an Electrochemical Concentration Cell (ECC) Ozonesonde up through the atmosphere while sending back data over a radio transmitter. With most ozone measurements reliant on sunlight, the ozonesondes flown from the South Pole are critical measurements during the six months of night at the bottom of the World. With the help of the Cooperative Agreement between NOAA and the University of Colorado (CIRES), these measurements will continue in to the foreseeable future.
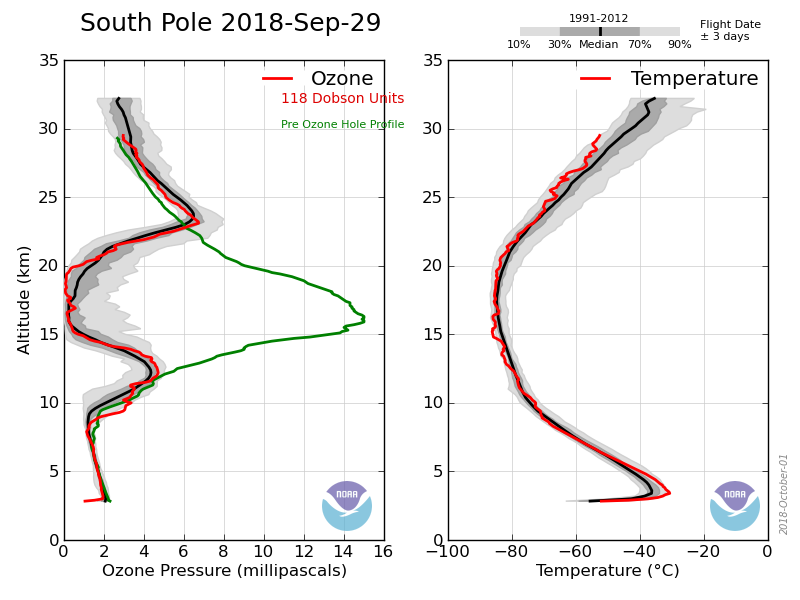
Directly measuring the air as the balloon rises, the ozonesondes produce the only highly detailed vertical profiles of atmospheric ozone from the surface to the edge of space. Sending back a new measurement once-per-second during the two and a half hour flight, the ozonesondes tell us exactly where the ozone is located and how intense the areas of depletion are progressing. Once the flight is complete, the data is transferred back to NOAA’s Global Monitoring Division in Boulder, Colorado where NOAA and CIRES researchers process and upload the profiles to public data archives for anyone to access and use in their research or studies.
