June 13th, 2016 – Determining Proper Dilution for Plating and Preparing LB Agar Plates
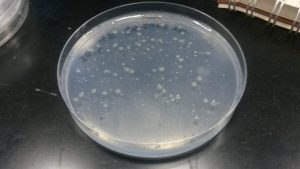 Today was a relatively fun day. The agar plates with various dilutions of soil bacteria from both the north-facing and south-facing slopes had incubated all weekend and thankfully had some growth on them. I was a bit surprised at how quickly the colonies formed! As expected, the plates with the smaller dilutions (10-4) had the most microbial diversity and growth while the plates with the larger dilutions (10-6) had very little. I was expecting that the intermediate dilution would be the best to use for the experiment, thinking that it would allow for the bacteria to be less crowded, but there was a notable decline in microbial diversity. If we dilute too much, we might have less accurate data as to the actual community composition. So… 10-4 it is!!
Today was a relatively fun day. The agar plates with various dilutions of soil bacteria from both the north-facing and south-facing slopes had incubated all weekend and thankfully had some growth on them. I was a bit surprised at how quickly the colonies formed! As expected, the plates with the smaller dilutions (10-4) had the most microbial diversity and growth while the plates with the larger dilutions (10-6) had very little. I was expecting that the intermediate dilution would be the best to use for the experiment, thinking that it would allow for the bacteria to be less crowded, but there was a notable decline in microbial diversity. If we dilute too much, we might have less accurate data as to the actual community composition. So… 10-4 it is!!
Last Friday, I had cleaned some more agar powder (about 50 g), placed it in a plastic bin, and Tess added a volatile gas to it so that it’d dry quickly over the weekend. When we checked on it today, it had dried quite a bit, but still retained some moisture. Tess commented that it was a nice preparation. The agar was very clean and not flaky. She assigned me to make more 1/100 LB agar plates by myself.
 I felt really confident in the procedure. I mixed all the solids with the proper amounts into two large plastic pitchers, added deionized water and was off to the autoclave machine to sterilize the solution. I left the solutions in the autoclave and went to my desk to research literature to determine how much of each resuscitation factor we needed to add to the plates for our experiment – information we’d need for tomorrow. After about 45 minutes, I checked on the LB agar solutions in the autoclave and headed back to the lab with them to let them cool. Upon observation, I noticed that the solids and DI water hadn’t mixed well. I determined that it would make sense to stir the solution up using magnetic stirrers but to my dismay I realized that I completely forgot to add the stirrers into the pitchers BEFORE autoclaving. This meant that I’d contaminate the solutions if I added them now. Darn it!
I felt really confident in the procedure. I mixed all the solids with the proper amounts into two large plastic pitchers, added deionized water and was off to the autoclave machine to sterilize the solution. I left the solutions in the autoclave and went to my desk to research literature to determine how much of each resuscitation factor we needed to add to the plates for our experiment – information we’d need for tomorrow. After about 45 minutes, I checked on the LB agar solutions in the autoclave and headed back to the lab with them to let them cool. Upon observation, I noticed that the solids and DI water hadn’t mixed well. I determined that it would make sense to stir the solution up using magnetic stirrers but to my dismay I realized that I completely forgot to add the stirrers into the pitchers BEFORE autoclaving. This meant that I’d contaminate the solutions if I added them now. Darn it!
I approached Tess about it and she told me that I’d need to autoclave them again with the stirrers included. She also pointed out that I forgot to add the autoclave tape to the pitchers which signify that they’re sterile. Oops. Not one but TWO mistakes today. I was not on my game!! Perhaps I got too overly confident.
Thankfully, these mistakes were simple to correct. I did as Tess instructed and added the stirrers, stirred the solutions until they dissolved, and autoclaved the solutions again with the stirrers and tape included. It turned out to be a blessing in disguise because the solutions came out much better than last time. They were homogeneous (the solids were evenly dissolved in solution)! Hooray!
I poured the solutions into the petri dishes and left them to dry overnight.
I’m glad that today worked out. I certainly won’t make the same silly mistakes again… though I wonder if this will be my last time making agar plates for a while.
